| єя«•«ьљƒ : ±Єњђ
|
ЅҐЉцєш»£ - 540059 141 |
| Multicenter Randomised Trial Evaluating the Efficacy of Cilostazol on Ischemic Vascular Complications after Drug-eluting Stent Implantation for Coronary Heart Disease (CILON-T trial) |
| ¬є мДЬмЪЄлМАнХЩкµРл≥СмЫР, ¬≤ лґДлЛємДЬмЪЄлМАнХЩкµРл≥СмЫР, ¬≥ к≥†лМАкµђл°Ьл≥СмЫР, вБік±імЦСлМАл≥СмЫР, 5 мґ©лґБлМАл≥СмЫР |
| ¬є мЭімКєнСЬ, ¬≤ мДЬм†ХмЫР, ¬є л∞Хк≤љмЪ∞, ¬є мЭінХімШБ, ¬є к∞ХнШДмЮђ, ¬є кµђл≥ЄкґМ, ¬≤ м°∞мШБмДЭ, ¬≤ мЧ∞нГЬмІД, ¬≤ м±ДмЭЄнШЄ, ¬≤ мµЬлПЩм£Љ, ¬≥ лЭЉмКємЪі, вБікґМнГЭкЈЉ, вБіл∞∞мЮ•нШЄ, 5 л∞∞мЮ•нЩШ, 5 м°∞л™Ем∞ђ, ¬є кєАнЪ®мИШ |
Background. The addition of cilostazol on the conventional dual antiplatelet therapy has been reported to reduce platelet activity and to improve clinical outcomes after percutaneous coronary intervention (PCI) in previous studies. We aimed to test whether cilostazol has similar beneficial effects in the real-world patients treated with intracoronary drug-eluting stent (DES).
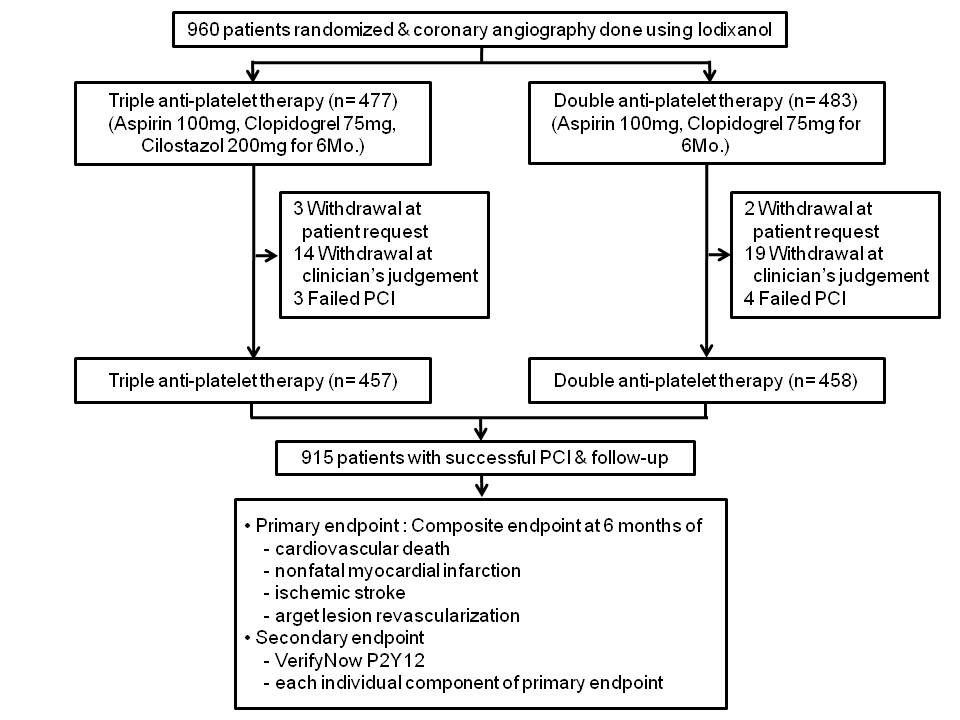
Methods. In a randomized multicenter trial, we enrolled 960 patients who received DES. They were randomized to receive either dual antiplatelet therapy (DAT; aspirin and clopidogrel) or triple antiplatelet therapy (TAT; aspirin, clopidogrel and cilostazol) for six months. Primary endpoint was the composite of cardiac death, nonfatal myocardial infarction (MI), ischemic stroke or target lesion revascularization (TLR). Secondary endpoints were P2Y12 reaction unit (PRU) measured with the VerifyNow P2Y12 assay at discharge and at six months after the index procedure. All cause of death, stent thrombosis, and each
component of primary endpoint at six month were another secondary endpoints. Analysis was done on an intention-to-treat basis. This study is registered with ClinicalTrials.gov, number NCT00776828.
Results. At six monthsвАЩ follow-up, there was no difference in the primary endpoint between two groups (8.5% in TAT vs. 9.2% in DAT, p=0.74). In secondary endpoint analysis, the TAT group achieved lower PRU levels than the DAT group both at discharge (206.6¬±90.3 vs. 232.2¬±80.3, p<0.001) and at six months (210.7¬±87.9 vs. 255.7¬±73.7, p<0.001). In the Cox proportional hazards analysis, lesion length (вЙ•28mm, HR 2.10, 95% CI 1.25~3.52) and PRU level at discharge (every increase in tertile, HR 1.61, 95% CI 1.16~2.25) were predictors of primary endpoint, but not the use of cilostazol (HR 0.90, 95% CI 0.54~1.52).
Conclusions. In spite of greater reduction of platelet reactivity by addition of cilostazol to conventional DAT, TAT did not show superiority in reducing the composite of adverse cardiovascular outcomes after DES implantation.
|
|
|
Warning: getimagesize(/home/virtual/circulationadmin/renewal/econgress/conference/abstract/img_files/1010CILON-Ttotal.jpg) [function.getimagesize]: failed to open stream: No such file or directory in /home/virtual/circulationadmin/new/econgress/conference/manage/schedule/view_abstract.php on line 164
|
|





