| єя«•«ьљƒ : ±Єњђ
|
ЅҐЉцєш»£ - 540532 5 |
| Intraindividual Variability of Residual Platelet Reactivity Assessed by VeryfyNow P2Y12 Assay in Patients Receiving Clopidogrel After Percutaneous Coronary Intervention |
| мЧ∞мДЄлМАнХЩкµР мЫРм£ЉмЭШк≥ЉлМАнХЩ мЫРм£ЉкЄ∞лПЕл≥СмЫР |
| мХИмД±кЈ†, мЭімКєнЩШ, мД±м§Ск≤љ, нХЬмГБмЪ∞, мЭім§АмЫР, мЬ§мШБмІД, кєАмЮ•мШБ, мЬ†л≥СмИШ, мЬ§м†ХнХЬ, мµЬк≤љнЫИ |
Background and Objectives: The evidence of an association of clopidogrel resistance with increased risk of thromboembolic events is gradually accruing and VeryfyNow (VN) P2Y12 point-of-care assay (Accumetrics, San Diego, California) has been increasingly utilizing to assess residual platelet reactivity in patients taking clopidogrel. No data concerning reproducibility of VN P2Y12 assay was found. The present study was aimed to measure agreement with repeated VN P2y12 reaction units (PRU) over time in the same patients with clopidogrel resistance.
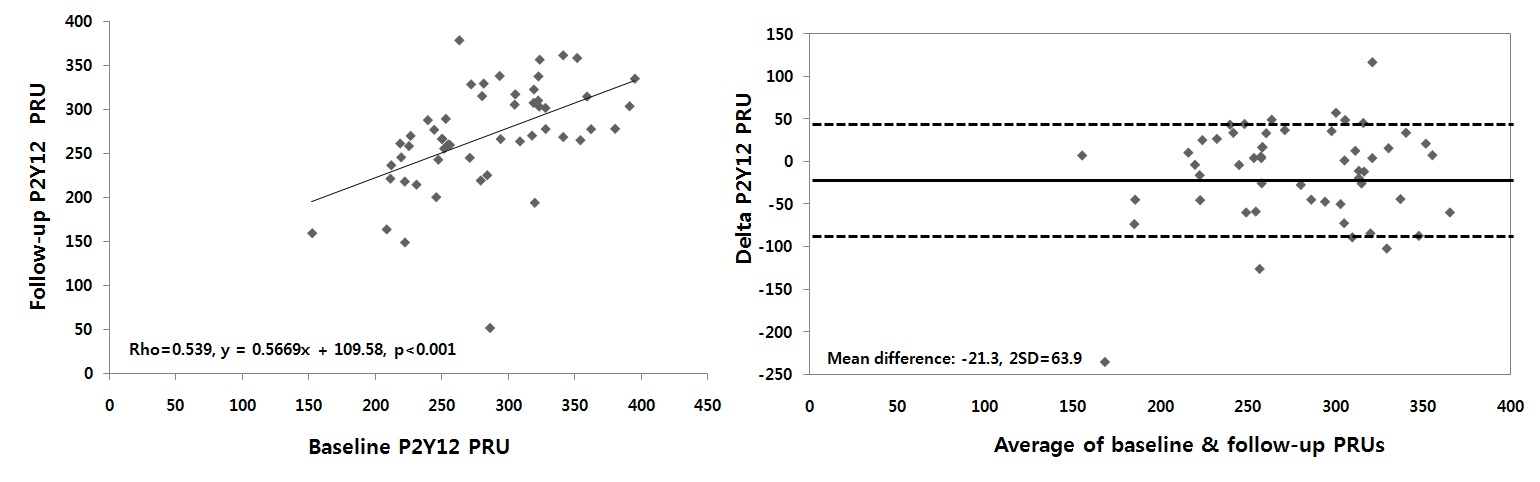
Methods: 50 patients (36 males, 66±11.2 years) with low response to clopidogrel were enrolled from 507 patients performed VN P2Y12 assay after PCI from Jan. 2006 to Dec. 2007 at our hospital. Low response to clopidogrel was defined as less than or equal to 20% inhibition of baseline PRU or more than or equal to 235 IU of PRU. VN P2Y12 assay was checked at 1 month following PCI and then 1 month from the initial measurement. Standard dual anti-platelet therapy (aspirin 100mg and clopidogrel 75mg) was maintained through the study. Agreement between the tests was assessed by Spearman rank correlation and Bland-Altman analysis.
Results: Baseline and follow-up PRUs were 209.2±49.2 and 268.9±63.3, respectively (mean difference -21.3±63.9, p=0.022). Initially, 43 (86%) patients were categorized into low response to clopidogrel in terms of both % inhibition and PRU. At follow-up, only 36 (72%) patients were low responders fulfilling both definitions. There was a fair correlation between baseline and follow-up PRU values (rho=0.539, figure left). The agreement between the 2 sets of measurements was not so high, with a mean difference of in PRU of -21.3±63.9 (figure right).
Conclusions: There is a considerable intraindividual variability of VN P2Y12 assay in patients receiving clopidogrel after PCI. Laboratory-determined clopidogrel resistance, assessed at a single time point, does not appear to be a stable phenomenon over time.
|
|
|
Warning: getimagesize(/home/virtual/circulationadmin/renewal/econgress/conference/abstract/img_files/IntraindividualVariability.jpg) [function.getimagesize]: failed to open stream: No such file or directory in /home/virtual/circulationadmin/new/econgress/conference/manage/schedule/view_abstract.php on line 164
|
|





