Arrhythmia 3
Genetic Variation Underlying Atrial Fibrillation Risk

Young Choi, MD, PhD
Catholic Univ., KoreaAtrial fibrillation (AF) is common cardiac condition affecting an estimated 2–3% of the global population. Increasing evidence indicates that genetic variation plays a pivotal role in determining individual susceptibility to AF. Both rare, highly penetrant mutations and common, small-effect variants contribute to its complex heritability, and advances in genomic technologies have enabled substantial progress in elucidating these mechanisms.
Earily studies of familial AF, or lone AF in large pedigrees, where rare variants with strong effects were identified in genes encoding cardiac ion channels and structural proteins. Mutations in KCNQ1, KCNA5, SCN5A, NPPA, TBX5, and PITX2, have been reported to cause Mendelian AF, often through altered atrial excitability or abnormal conduction. In addition, variants in sarcomeric and cytoskeletal genes, such as MYH6, TTN and LMNA, highlight the contribution of atrial structural remodeling and cardiomyopathy-related processes to AF risk.
On the other hand, in most patients with AF, such pathogenic mutations are not observed, and common genetic variants are considered to play a more significant role in genetic susceptibility to AF. Previous GWAS on AF has identified approximately 900 SNPs, and the latest large-scale cross-ancestry GWAS, which included 14,554 AF cases and 2,193,634 controls, reported 146 SNPs associated with AF. The development of polygenic risk scores (PRS) has enabled quantification of the cumulative impact of common variants. Recent studies demonstrate that PRS can stratify AF risk independently of clinical predictors and may identify individuals at particularly high lifetime risk even in the absence of conventional risk factors. Integration of PRS into risk prediction models may improve screening strategies, guide preventive measures, and potentially influence therapeutic decision-making, such as early rhythm-control interventions. However, the transferability of PRS across ethnic groups remains limited due to Eurocentric bias in existing GWAS datasets, highlighting the need for larger studies in other ethnic populations, such as East Asia.
Despite this progress, significant challenges remain. The overall heritability of AF is estimated at 20–30%, indicating that many genetic and environmental contributors are yet to be discovered. Epigenetic regulatory mechanisms, including non-coding RNAs, DNA methylation, and histone modifications, have also been implicated in the pathogenesis of AF. Currently, the role of underlying genetic variation in AF remains incompletely understood, and such research is especially scarce in Korea, where greater contributions from investigators are expected.
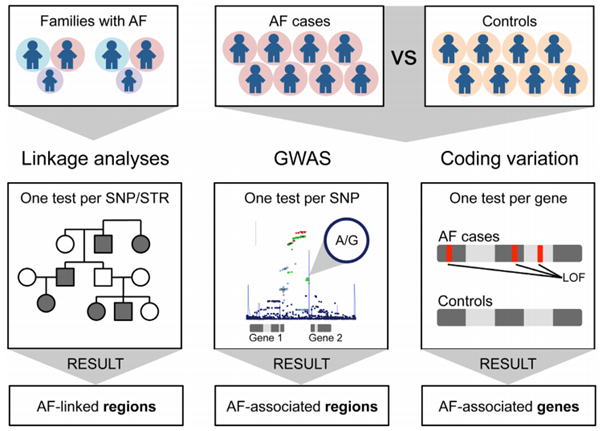
Roselli et al, Genetics of Atrial fibrillation in 2020, Circulation Research 2020
